5 Steps of EBP
Use the five "A's" to remember the critical steps of the evidence-based practice process:
ASK the answerable clinical question.
ACQUIRE the most relevant and best evidence to answer the question.
APPRAISE the evidence critically for validity, relevance, and applicability.
APPLY the evidence, along with critical expertise and the patient's preferences and values.
ASSESS the effectiveness and efficiency of the previous four steps and seek ways to improve one's ability to ask, acquire, appraise, and apply.
This guide covers the first 3 steps.
Asking the Question
The "Well-Built Clinical Question"
PICO(T)
The first part of any research is identifying the question you want to answer. This is very important because the more you understand your question the more likely you are to obtain relevant results. The process of formulating a good search question is known in evidence-based health care as “the well-built clinical question.”* One way of building your search question starts with the patient and is known as PICO, which stands for:
- P - Patient or Population or Problem/Disease
- Who or what is the question about? This may include the primary problem, disease, or circumstances. Sometimes the sex, age, or race of a patient might be relevant to the diagnosis or treatment of a disease.
- I - Intervention, Exposure or Prognostic Factor
- What main intervention/treatment are you considering? What factor may influence the prognosis of the patient, such as age or comorbidities? What was the patient exposed to?
- C - Comparison(s) or Control
- What alternative intervention are you considering, if any? For example, you might be comparing the efficacy of two medications or the accuracy of two diagnostic tests. Your clinical question does not have to always have a specific comparison.
- O - Outcome(s)
- What are you trying to accomplish or measure? What are you trying to do for the patient or problem? Examples might include managing a disease, alleviating symptoms, preventing a disease, etc.
- T - Timeframe (optional)
- What's the amount of time that you'll be observing the patient or problem. For example, improving rates of hospital-acquired infections over the course of a year.
Also consider the two Ts
Type of Question
- Diagnosis : How to select and interpret diagnostic tests
- Therapy : How to select treatments to offer patients that do more good than harm and that are worth the efforts and costs of using them
- Prognosis : How to estimate the patient’s likely clinical course over time and anticipate likely complications of disease
- Etiology : How to identify causes for disease, including genetics
Type of Study
Evidence Hierarchy
The evidence hierarchy pyramid is a visual representation of the strength of different research study designs. It can be helpful to think about evidence as a pyramid – not all study designs and resource types are created equal.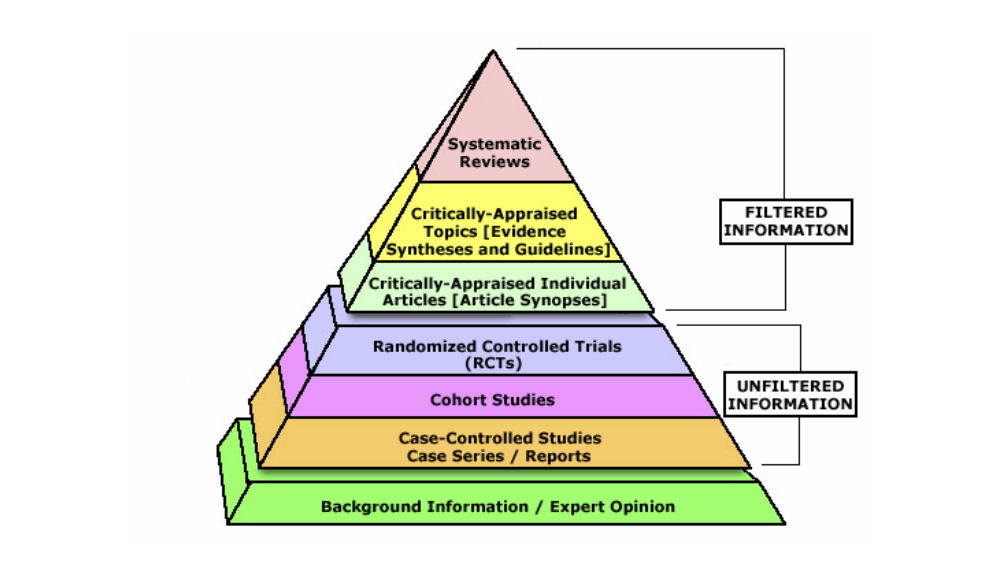
Filtered Information
At the top of the pyramid, we have filtered information – this includes systematic reviews, meta-analyses, and evidence syntheses; practice guidelines; and critically-appraised topics found in clinical resources. This type of information has used a high-quality methodology to synthesize primary resources – meaning that they have searched for available primary literature and evaluated its validity to provide answers to specific clinical questions. It is important to remember that the quality and reliability of filtered information can only be as good as the primary literature it includes.
Unfiltered Information
In the middle of the pyramid, we have unfiltered information – this is known as primary literature. These are individual experimental study designs. A randomized controlled trial is considered the highest quality individual study design, followed by cohort studies and case-controlled studies. We will discuss these study designs in more detail later in the tutorial.
Background Information
At the base of the pyramid, we have background information and expert opinion. Background information is not typically used in making complex clinical decisions, but can be helpful in defining parts of your clinical question.
Acquiring the Evidence
Synthesized resources
Start with:
- DynaMedAn evidence-based clinical decision support tool. Contains over 3,000 topic summaries covering diseases, disorders, diagnostics, and drugs with extensive references to journals and practice guidelines.
- Cochrane LibraryThe Cochrane Library consists of a collection of regularly updated evidence-based health care databases. The Cochrane collection is designed to provide information and evidence to support health care decision-making.
- Natmed Pro (previously titled Natural Medicines)Natural Medicines provides evidence-based information and ratings on over 90,000 dietary supplements, natural medicines, and integrative therapies. Includes these databases: Foods, Herbs, & Supplements; Health & Wellness; Sports Medicine; Comparative Effectiveness; Manufacturers; Commercial Products; and Medical Conditions.
Primary article databases
Then try these:
- PubMedSearches MEDLINE, which is the primary source of journal articles for the health sciences (fields of medicine, nursing, dentistry, veterinary medicine, public health, health care systems, and basic sciences). Coverage is from the 1940s to the present. View this tutorial to learn how to go from a general idea to a very precise set of results of journal articles and scholarly materials.
- Ovid MEDLINESearches MEDLINE, which is the primary source of journal articles for the health sciences (fields of medicine, nursing, dentistry, veterinary medicine, public health, health care systems, and basic sciences). Ovid MEDLINE is optimized for advanced literature searches. Coverage is from the 1940s to the present.
- CINAHL Ultimate (Nursing & Allied Health)Covers nursing and allied health journal articles, book chapters, and dissertations, as well as providing summarized evidence-based resources such as care sheets and quick lessons.
- Embase / Embase ClassicEmbase is a biomedical and pharmacological database covering journal articles, conference proceedings, and gray literature. It is strong in its coverage of pharmaceutical research and international and non-English content. Covers 1947 to present.
- TRIP DatabaseThe TRIP database contains evidence-based health care resources, such as guidelines, systematic reviews, and primary research. Each abstract denotes the source and description of information along with complete citations.
Clinical Practice Guidelines
Also consider these specialized resources:
Additional Resources
Books
How to Read a Paper by
ISBN: 9781119484721Publication Date: 2019-04-04Required reading in many medical and healthcare institutions, How to Read a Paper is a clear and wide-ranging introduction to evidence-based medicine and healthcare, helping readers to understand its central principles, critically evaluate published data, and implement the results in practical settings. Author Trisha Greenhalgh guides readers through each fundamental step of inquiry, from searching the literature to assessing methodological quality and appraising statistics. How to Read a Paper addresses the common criticisms of evidence-based healthcare, dispelling many of its myths and misconceptions, while providing a pragmatic framework for testing the validity of healthcare literature. Now in its sixth edition, this informative text includes new and expanded discussions of study bias, political interference in published reports, medical statistics, big data and more. Offers user-friendly guidance on evidence-based healthcare that is applicable to both experienced and novice readers Authored by an internationally recognised practitioner and researcher in evidence-based healthcare and primary care Includes updated references, additional figures, improved checklists and more How to Read a Paper is an ideal resource for healthcare students, practitioners and anyone seeking an accessible introduction to evidence-based healthcare.
Toolkits
- Centre for Evidence-Based MedicineCEBM is a great resource both for its own content and links to other EBP sites. Under EBM Tools you can find critical appraisal worksheets, calculators, and guides for finding the best evidence. Resources provides copies of EBP presentations (which are you free to reuse), workshop videos, and links to articles and other EBP groups on the web.
- Knowledge Translation ProgramAn Evidence-Based Medicine toolkit from the Knowledge Translation Program in Canada.
Tutorial
- Tutorial: Evidence Based Practice: An interprofessional tutorialAn interactive, self-paced orientation to foundational evidence-based practice methodology and skills. This tool features case studies from across the health professions, with an emphasis on finding and critically appraising best evidence.
